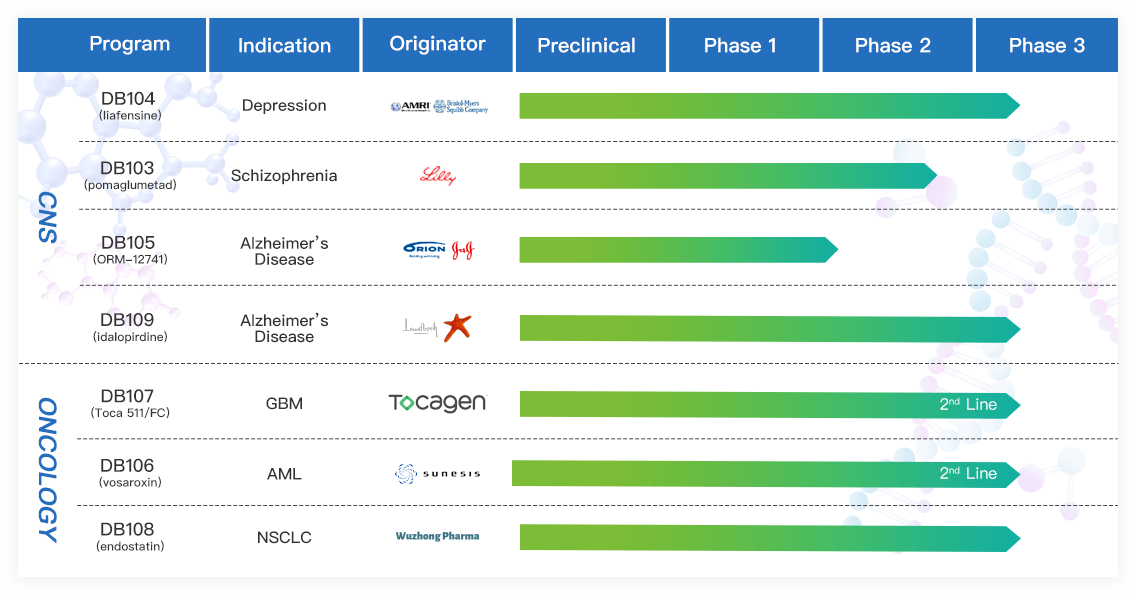
Denovo Biopharma is a clinical-stage biopharmaceutical company focused on the development of biomarker-driven precision medicines. Our technology platform can be broadly applied to biomarker discovery and biomarker-guided drug development in many therapeutic areas such as oncology, neurology, metabolic, cardiology, and immunology.
Our core technology is the industry's first platform and algorithm to perform de novo genomic biomarker discovery retrospectively using archived clinical samples. This technology is especially useful for late-stage drugs that have completed clinical trials with unsatisfactory efficacy. By identifying biomarkers correlated to patients' responsiveness to drug candidates retrospectively, our technology enables new clinical trials in a targeted patient population to achieve significant efficacy and/or less adverse effects.